For patients on hemodialysis: 2-year, real-world data
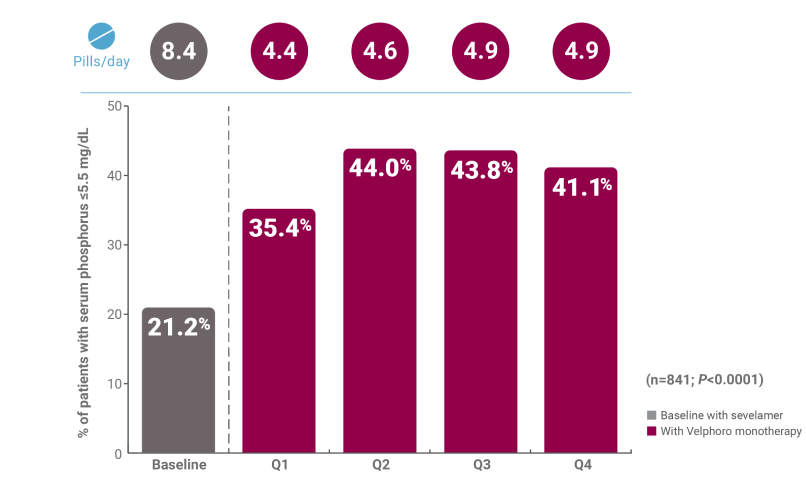
Patients on hemodialysis switched from sevelamer: 1-year, real-world data
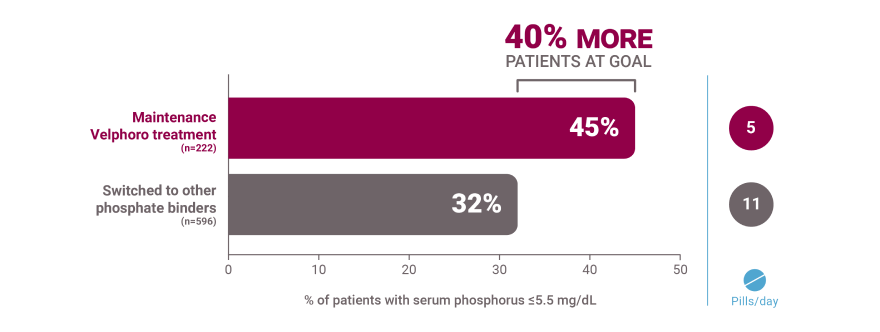
Real-world data overview of patients on hemodialysis
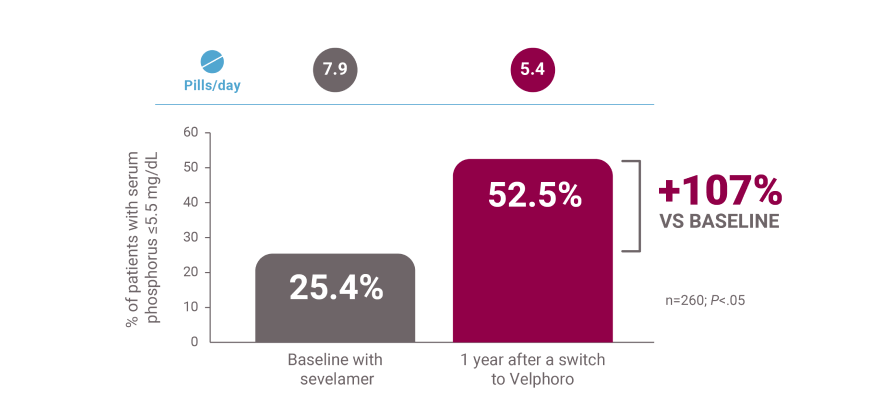
Patients on peritoneal dialysis (PD) switched from sevelamer: 1-year, real-world data
Clinical trial data
Tablets not actual size.
*A retrospective analysis of deidentified clinical and prescription data of 818 hemodialysis patients extracted from the Fresenius Kidney Care clinical data warehouse and Fresenius pharmacy database. Clinical and laboratory parameters of 222 patients who continued Velphoro monotherapy for 24 months from baseline beginning between April 1, 2014 and April 1, 2015 were compared to 596 patients who discontinued Velphoro and were switched to alternate phosphate binders within 90 days of baseline. The decision to switch phosphate binders was made on a clinical basis and the reasons underlying this change were not available. Outcomes included achievement of target serum phosphorus of ≤5.5 mg/dL and phosphate binder pill burden.1
†A retrospective analysis of deidentified pharmacy data from 1,792 adult in-center hemodialysis patients who were switched to Velphoro during routine care between May 2018 and May 2019. Subsets evaluated included patients taking ferric citrate at baseline (n=115) and those taking sevelamer at baseline (n=841). The decision to discontinue baseline phosphate binders (PBs) and switch to Velphoro was made on a clinical basis and the reasons underlying this change were not available. Comparisons were made between the 91-day period before Velphoro initiation (ie, baseline) and the 4 consecutive 91-day intervals of Velphoro treatment (Q1-Q4). Main outcome measures included achievement of target phosphorus levels (≤5.5 mg/dL) and mean number of PB pills/day.3
‡A retrospective analysis of deidentified pharmacy data from 4,925 adult in-center hemodialysis patients who were switched to Velphoro during routine care between April 2014 and January 2017. The decision to switch phosphate binders (PBs) was made on a clinical basis and the reasons underlying this switch were not available. Outcomes included the proportion of patients with phosphorus levels ≤5.5 mg/dL and the mean prescribed PB pills/day at baseline (3 months prior to Velphoro) and during Velphoro follow-up (up to 2 years after switch to Velphoro).4
A retrospective analysis of deidentified pharmacy data from 1,029 adult in-center hemodialysis patients who were switched to Velphoro during routine care between April 2014 and March 2015. The decision to switch phosphate binders (PBs) was made on a clinical basis and the reasons underlying this change were not available. Outcomes included the proportion of patients with phosphorus levels ≤5.5 mg/dL and the mean prescribed PB pills/day at baseline (3 months prior to Velphoro) and during Velphoro follow-up (6 months after switch to Velphoro, n=424).5
A retrospective analysis of deidentified pharmacy data from 530 adult in-center hemodialysis patients who were switched to Velphoro during routine care between March 2014 and March 2015. The decision to discontinue baseline phosphate binders (PBs) and switch to Velphoro was made on a clinical basis and the reasons underlying this change were not available. Comparisons were made between the 91-day period before Velphoro initiation (ie, baseline) and the 4 consecutive 91-day intervals of Velphoro treatment (Q1-Q4). Main outcome measures included achievement of target phosphorus levels (≤5.5 mg/dL) and mean number of PB pills/day.6
A retrospective analysis of deidentified clinical and prescription data from 241 adult in-center hemodialysis patients who were switched to Velphoro during routine care. The decision to switch phosphate binders (PBs) was made on a clinical basis and the reasons underlying this change were not available. Outcomes included the proportion of patients with phosphorus levels ≤5.5 mg/dL and mean prescribed PB pills/day at baseline (3 months prior to Velphoro) and 24 months of Velphoro treatment.7
A switch to Velphoro demonstrated a 95% increase in patients achieving KDOQI goals.5
§Presenter is a paid speaker and has been compensated for their time.
‖A retrospective analysis of deidentified clinical and prescription data from 260 patients on peritoneal dialysis extracted from the Fresenius Kidney Care clinical data warehouse and Fresenius pharmacy database. Patients were switched from another phosphate binder to Velphoro monotherapy between May 2018 and May 2019, received another phosphate binder therapy and had available serum phosphorus during 3 months before Velphoro prescriptions, and had 1 year of uninterrupted Velphoro prescriptions. Comparisons were made between baseline (3 months before Velphoro prescription) and 4 consecutive 91-day intervals through 12 months of Velphoro prescriptions. Outcomes included achievement of in-range serum phosphorus (≤5.5 mg/dL) and number of phosphate binder pills per day.8
¶A 52-week, open-label, active-controlled, phase 3 study evaluated the safety and efficacy of Velphoro in lowering serum phosphorus levels in patients (N=1,054) with chronic kidney disease on hemodialysis or peritoneal dialysis. In the titration phase (first 24 weeks), patients were randomized to receive either Velphoro or sevelamer carbonate to establish the noninferiority of Velphoro to sevelamer carbonate in lowering serum phosphorus at 12 weeks (secondary endpoint). The following withdrawal phase (weeks 24 to 27, n=93) established the superiority of Velphoro with an effective maintenance dose over a placebo-like low dose (primary endpoint). During a final long-term maintenance phase (weeks 28 to 52, n=658), patients continued phosphate binder treatment according to their original randomization for the assessment of long-term efficacy, safety, and tolerability.2,10
#Over the course of the 52-week study, patients on Velphoro took an average of 5.4 fewer tablets per day: 5.4 x 365 = 1,971.10
References: 1. Coyne DW, Ficociello LH, Parameswaran V, et al. Sucroferric oxyhydroxide in maintenance hemodialysis: a retrospective, comparative cohort study. Kidney Med. 2020;2(3):307-316. 2. Velphoro® [package insert]. Waltham, MA: Fresenius Medical Care North America; 2024. 3. Kendrick JB, Zhou M, Ficociello LH, et al. Serum phosphorus and pill burden among hemodialysis patients prescribed sucroferric oxyhydroxide: one-year follow-up on a contemporary cohort. Int J Nephrol Renovasc Dis. 2022;15:139-149. 4. Coyne DW, Ficociello LH, Parameswaran V, et al. Effectiveness of sucroferric oxyhydroxide in lowering serum phosphorus in 4,925 chronic hemodialysis patients. ASN Kidney Week 2017. New Orleans, Louisiana: October 31-November 5, 2017. Poster TH-P01031. 5. Coyne DW, Ficociello LH, Parameswaran V, et al. Real-world effectiveness of sucroferric oxyhydroxide in patients on chronic hemodialysis: a retrospective analysis of pharmacy data. Clin Nephrol. 2017;88(2):59-67. 6. Kendrick J, Parameswaran V, Ficociello LH, et al. One-year historical cohort study of the phosphate binder sucroferric oxyhydroxide in patients on maintenance hemodialysis. J Ren Nutr. 2019;29(5):428-437. 7. Sprague SM, Parameswaran V, Ficociello LH, et al. Two year follow up on chronic hemodialysis patients prescribed sucroferric oxyhydroxide as part of routine care. American Society of Nephrology: Kidney Week 2017; October 31-November 5, 2017. New Orleans, LA. 8. Kalantar-Zadeh K, Ficociello L, Zhou M, Parameswaran V, Mullan C, Anger M. Management of serum phosphorus (sP) over one-year follow up in peritoneal dialysis (PD) patients prescribed sucroferric oxyhydroxide (SO) as part of routine care. American Kidney Association 2021. San Diego, California: November 4-7, 2021. Poster P00545. 9. Data on file. Fresenius Medical Care North America, Waltham, MA. 10. Floege J, Covic AC, Ketteler M, et al; on behalf of the Sucroferric Oxyhydroxide Study Group. Long‐term effects of the iron‐based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30(6):1037‐1046.