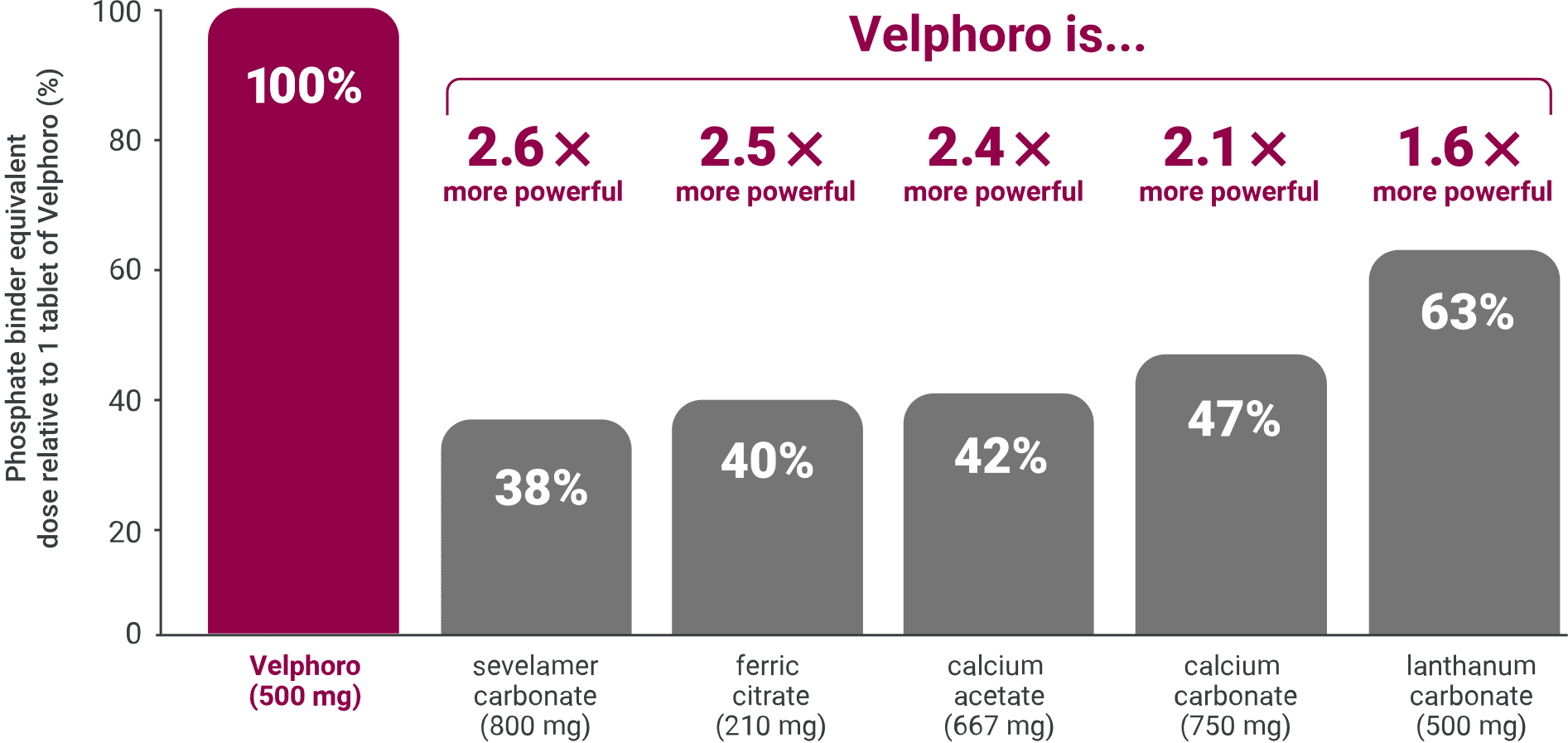
Velphoro is 2.6x more powerful than 1 tablet of sevelamer*1
*Based on an analysis of comparative clinical studies that roughly established an equivalent dose for phosphate binders relative to the phosphate binding capacity of calcium carbonate.1
More than 40% of patients are not at goal, indicating the ongoing challenge of phosphate management4-7
DOPPS=Dialysis Outcomes and Practice Patterns Study
KDIGO=Kidney Disease: Improving Global Outcomes
KDOQI=Kidney Disease Outcomes Quality Initiative
References: 1. Coyne DW, Larson DS, Delmez JA. Bone disease. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 5th ed. Wolters Kluwer Health; 2015:665-692. 2. Wilhelm M, Gaillard S, Viatcheslav R, Funk F. The iron-based phosphate binder PA21 has potent phosphate binding capacity and minimal iron release across a physiological pH range in vitro. Clin Nephrol. 2014;(81)4:251-258. 3. Velphoro® [package insert]. Waltham, MA: Fresenius Medical Care North America; 2024. 4. Dialysis Outcomes and Practice Patterns Study Program. DOPPS Practice Monitor. Serum phosphorus (most recent) categories. https://www.dopps.org/DPM/Files/phosphmgdl_c_overallTAB.htm. Accessed May 25, 2021. 5. Kidney Disease: Improving Global Outcomes. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2017;7(suppl 1):1-59. 6. Kidney Disease: Improving Global Outcomes. KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009;76(suppl 113):1-140. 7. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1-S201. 8. Dialysis Outcomes and Practice Patterns Study Program. DOPPS Practice Monitor. Phosphate binder use, by type. https://www.dopps.org/DPM/Files/pbgroup_c_overallTAB.htm. Accessed May 25, 2021. 9. Data on file. Fresenius Medical Care North America, Waltham, MA.