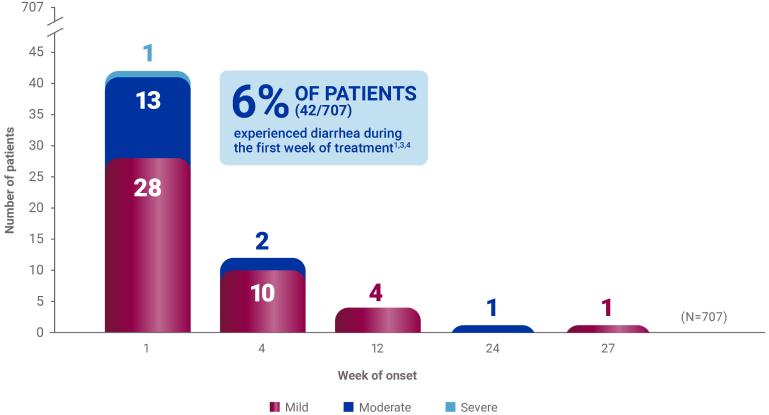
*A 52-week, open-label, active-controlled, phase 3 study evaluated the safety and efficacy of Velphoro in lowering serum phosphorus levels in patients (N=1,054) with chronic kidney disease on hemodialysis or peritoneal dialysis. In the titration phase (first 24 weeks), patients were randomized to receive either Velphoro or sevelamer carbonate to establish the noninferiority of Velphoro to sevelamer carbonate in lowering serum phosphorus at 12 weeks (secondary endpoint). The following withdrawal phase (weeks 24 to 27, n=93) established the superiority of Velphoro with an effective maintenance dose over a placebo-like low dose (primary endpoint). During a final long-term maintenance phase (weeks 28-52, n=658), patients continued phosphate binder treatment according to their original randomization for the assessment of long-term efficacy, safety, and tolerability.1
References: 1. Velphoro® [package insert]. Waltham, MA: Fresenius Medical Care North America; 2024. 2. Floege J, Covic AC, Ketteler M, et al: on behalf of the Sucroferric Oxyhydroxide Study Group. Long‐term effects of the iron‐based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30(6):1037‐1046. 3. Floege J, Covic AC, Ketteler M, et al; on behalf of the PA21 Study Group. A phase III study of the efficacy and safety of a novel iron‐based phosphate binder in dialysis patients. Kidney Int. 2014;86(3):638-647. 4. Data on file. Fresenius Medical Care North America, Waltham, MA. 5. Renvela [package insert]. Cambridge, MA: Genzyme Corporation, a Sanofi Company; 2023. 6. PhosLo [package insert]. Waltham, MA: Fresenius Medical Care North America; 2011. 7. Auryxia [package insert]. Cambridge, MA: Akebia Therapeutics, Inc.; 2024. 8. Fosrenol [package insert]. Lexington, MA: Takeda Pharmaceuticals America, Inc.; 2024. 9. Renagel [package insert]. Cambridge, MA: Genzyme Corporation; 2021.