When given a choice to chew Velphoro or break and swallow†3:
Recommended
starting dose
Titrate by 1 tablet per day,
as required
Maintain
phosphorus control
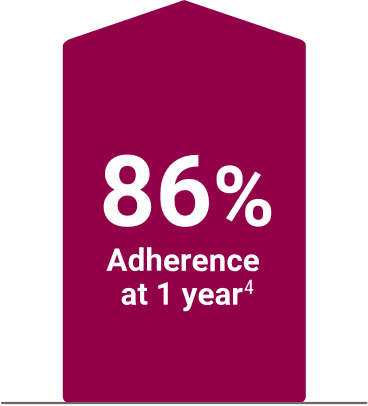
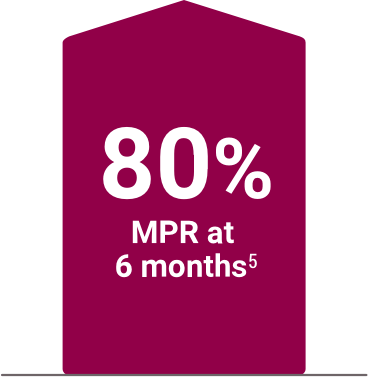
High adherence demonstrated in clinical and real-world settings4,5
Medication possession ratio (MPR) in a 6-month,
real-world analysis of pharmacy service data (n=30)§5
*Presenter is a paid speaker and has been compensated for their time.
†Prospective analysis of 105 patients on hemodialysis to assess acceptance and strategies that support compliance in clinical practice. All patients were on a phosphate binder: at baseline, 57 were switched to Velphoro and 48 maintained their baseline treatment. Patients were informed about various modes of administration and were permitted to adapt administration based on preference.3
‡A 52-week, open-label, active-controlled, phase 3 study evaluated the safety and efficacy of Velphoro in lowering serum phosphorus levels in patients (N=1,054) with chronic kidney disease on hemodialysis or peritoneal dialysis. In the titration phase (first 24 weeks), patients were randomized to receive either Velphoro or sevelamer carbonate to establish the noninferiority of Velphoro to sevelamer carbonate in lowering serum phosphorus at 12 weeks (secondary endpoint). The following withdrawal phase (weeks 24 to 27, n=93) established the superiority of Velphoro with an effective maintenance dose over a placebo-like low dose (primary endpoint). During a final long-term maintenance phase (weeks 28 to 52, n=658), patients continued phosphate binder treatment according to their original randomization for the assessment of long-term efficacy, safety, and tolerability.1
§A retrospective analysis of deidentified pharmacy data from adult hemodialysis patients (n=490) who were enrolled with the DaVita pharmacy service for at least 6 months. Subjects were patients who converted to Velphoro monotherapy from another phosphate binder during routine care between April 2014 and September 2016. The decision to switch phosphate binders (PBs) was made on a clinical basis and the reasons underlying this change were not available. Primary outcomes were total daily PB pill burden, total PB medication possession ratio, serum phosphorus, and percentage of patients with serum phosphorus ≤5.5 mg/dL.5
References: 1. Velphoro® [package insert]. Waltham, MA: Fresenius Medical Care North America: 2024. 2. Lanz M, Baldischweiler J, Kriwet B, Schill J, Stafford J, Imanidis G. Chewability testing in the development of a chewable tablet for hyperphosphatemia. Drug Dev Ind Pharm. 2014;40(12):1623-1631. 3. Arenas Jiménez MD, Navarro González JF. How to improve adherence the captors of phosphorus on hemodialysis: experience in real life with sucroferric oxyhydroxide. Nefrologia (Engl Ed). 2020;40(6):640-646. 4. Floege J, Covic AC, Ketteler M, et al; on behalf of the Sucroferric Oxyhydroxide Study Group. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30(6):1037-1046. 5. Gray K, Ficociello LH, Hunt AE, Mullon C, Brunelli SM. Phosphate binder pill burden, adherence, and serum phosphorus control among hemodialysis patients converting to sucroferric oxyhydroxide. Int J Nephrol Renovasc Dis. 2019;12:1-8. 6. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117-122.