Resources
Frequently asked questions
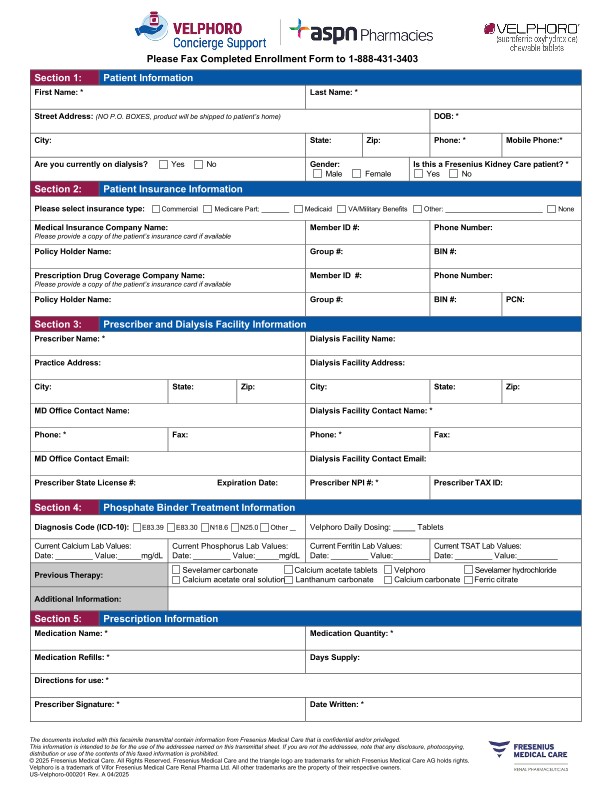
The process of getting Velphoro has been simplified for the majority of patients. The Medicare Prospective Payment System* helps ensure equitable access to Velphoro for patients on dialysis. Each dialysis organization has a customized formulary that will mean minimal paperwork for you and your staff. There's no additional copay or coinsurance for most patients,† and prescriptions can be delivered by mail, which may help adherence to Velphoro treatment.1
For patients whose phosphate binders are not covered through the Medicare Prospective Payment System,* help is available through Velphoro Concierge Support, powered by ASPN Pharmacies. Details about ways to prescribe and the benefits of Velphoro Concierge Support can be found on the Access page.
Velphoro has no interaction with commonly prescribed oral drugs, including oral calcitriol. Velphoro can be administered with levothyroxine, as long as the doses are separated by 4 hours. Take acetylsalicylic acid, cephalexin, and doxycycline at least 1 hour before Velphoro. For medications where a reduction in bioavailability would have significant effect, consider separating the administration of the two drugs, taking into account absorption characteristics of the second medication.2
There are no contraindications for Velphoro.2
Velphoro is the most potent phosphate binder per tablet.3 Use of Velphoro has been shown to reduce the phosphate-binder pill burden for patients on dialysis by half, while doubling the percentage of patients who achieve serum phosphorus levels of ≤5.5 mg/dL.4,5
Velphoro is a berry-flavored chewable tablet, not intended to be swallowed whole. Velphoro can also be broken or crushed. The initial starting dose for Velphoro is one 500-mg tablet, 3 times a day, with meals. Monitor serum phosphorus levels and titrate the dose of Velphoro by 1 tablet per day (not per meal), as often as weekly.2
Velphoro must be administered with meals. If a dose is missed, resume Velphoro with the next meal.2
In vitro studies have shown that up to 96% of available phosphate is bound by Velphoro, then eliminated in the feces. Reduction in serum phosphorus and calcium-phosphorus product levels results from reduced dietary phosphate absorption.2
Adverse events with Velphoro are usually mild and transient, and similar to those of sevelamer, as seen in a 55-week, open-label, active-controlled, parallel-design safety and efficacy study of 968 hemodialysis patients, and 86 peritoneal dialysis patients. Adverse reactions in >5% of patients taking Velphoro included diarrhea (24%), discolored feces (16%), and nausea (10%). The majority of cases of diarrhea were mild, generally occurred early in treatment, and resolved with continued use. Additional reported reactions include tooth discoloration and rash.2
Velphoro® (sucroferric oxyhydroxide) is a phosphate binder indicated for the control of serum phosphorus levels in adult and pediatric patients 9 years of age and older with chronic kidney disease on dialysis.2
*As of January 1, 2025, phosphate binders are included in the End-Stage Renal Disease (ESRD) Prospective Payment System, and Medicare beneficiaries with ESRD for whom phosphate binders are medically indicated will receive their phosphate binder medication through their dialysis provider. Certain patients, such as those for whom Medicare eligibility is pending, certain Medicare Advantage beneficiaries, and those whose commercial insurance plans are the primary payer, may not be eligible to receive their phosphate binder through their dialysis organization and may still have a choice of pharmacy.
†Includes fee-for-service patients receiving phosphate binder therapy through their dialysis organization. Out-of-pocket costs are based on the patient's medical health plan coinsurance structure.
References: 1. Centers for Medicare & Medicaid Services. Medicare Program; End-Stage Renal Disease Prospective Payment System, Payment for Renal Dialysis Services Furnished to Individuals With Acute Kidney Injury, Conditions for Coverage for End-Stage Renal Disease Facilities, End-Stage Renal Disease Quality Incentive Program, and End-Stage Renal Disease Treatment Choices Model. Fed Regist. 2024;89(218):89084-89213. 42 CFR Parts 410, 413, 494, and 512. https://www.govinfo.gov/content/pkg/FR-2024-11-12/pdf/2024-25486.pdf 2. Velphoro® [package insert]. Waltham, MA: Fresenius Medical Care North America; 2024. 3. Coyne DW, Larson DS, Delmez JA. Bone disease. In: Daugirdas JT, Blake PG, Ing TS, eds. Handbook of Dialysis. 5th ed. Wolters Kluwer Health; 2015:665-692. 4. Coyne DW, Ficociello LH, Parameswaran V, et al. Real-world effectiveness of sucroferric oxyhydroxide in patients on chronic hemodialysis: a retrospective analysis of pharmacy data. Clin Nephrol. 2017;88(2):59-67. 5. Kendrick J, Parameswaran V, Ficociello LH, et al. One-year historical cohort study of the phosphate binder sucroferric oxyhydroxide in patients on maintenance hemodialysis. J Ren Nutr. 2019;29(5):428-437.